principle of barfoed test|Barfoed's test : Tagatay Barfoed’s test is a chemical test used to detect the presence of monosaccharides which detects reducing monosaccharides in the presence of disaccharides. This reaction . Tingnan ang higit pa Click here to Register! USERNAME PASSWORD: Forgot your user ID / password?
PH0 · Experiment
PH1 · Carbohydrates
PH2 · Barfoed’s Test: Principle, Reagents & Result Interpretation
PH3 · Barfoed’s Test: Principle, Procedure, Reaction, and Result
PH4 · Barfoed’s Test: Objective, Principle, Reagents,
PH5 · Barfoed’s Test: Objective, Principle, Procedure, Results
PH6 · Barfoed’s Test
PH7 · Barfoed's test
PH8 · Barfoed's Test
Elegibilidade: O serviço essencial de monitoramento de identidade da McAfee® está disponível nas assinaturas ativas do McAfee+ Premium, McAfee+ Advanced, McAfee+ Ultimate, McAfee Total Protection e McAfee LiveSafe. Nem todos os elementos de monitoramento de identidade estão disponíveis em todos os países.
principle of barfoed test*******The Barfoed reagent is made up of copper acetate in a dilute solution of acetic acid. Since acidic pH is unfavorable for reduction, monosaccharides, which are strong reducing agents, react in about 1-2 min. However, the reducing disaccharides take a . Tingnan ang higit paImage Reaction Source: Chemistry Learner, Created with BioRender.com. 1. The presence of red precipitate detects the presence . Tingnan ang higit pa
Barfoed’s test is a chemical test used to detect the presence of monosaccharides which detects reducing monosaccharides in the presence of disaccharides. This reaction . Tingnan ang higit paBarfoed’s test is a chemical test used for detecting the presence of monosaccharides. It is based on the reduction of cupric (II) acetate to cuprous (I) oxide (Cu 2 O), which forms a brick-red precipitate. Barfoed’s test is a biochemical test used to detect monosaccharide (reducing) sugars in solution. The technique was devised by a Swedish physician C. .Barfoed's test is a chemical test used for detecting the presence of monosaccharides. It is based on the reduction of copper(II) acetate to copper(I) oxide (Cu2O), which forms a brick-red precipitate. RCHO + 2Cu + 2H2O → RCOOH + Cu2O↓ + 4H (Disaccharides may also react, but the reaction is much slower.) The aldehyd.
Barfoed's test Principle. Barfoed’s test reaction is based on the reduction of cupric acetate by reducing monosaccharides and reducing disaccharides. Reduction of cupric .Barfoed’s test is a chemical test used to distinguish between monosaccharides and disacchar ides according to their capacity to generate copper(I) oxide (Cu 2 O) in an .
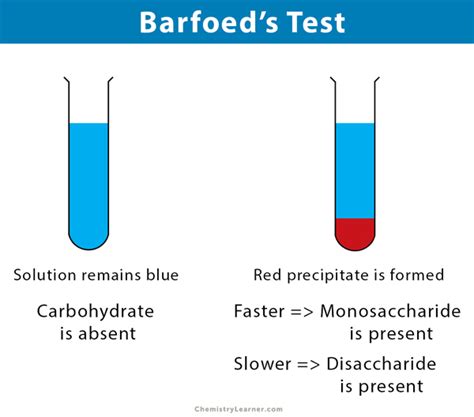
Principle of Barfoed’s test: Barfoed’s test is used for distinguishing monosaccharides from reducing disaccharides. Monosaccharides usually react in about 1-2 minute while the reducing . Barfoed’s Test In this part of the experiment, you will again test known samples of glucose, fructose, lactose, sucrose, starch, and compare with a sample of a .Shows positive test for: Reducing monosaccharides Reactions: Reducing monosaccharides are oxidized by the copper ion in solution to form a carboxylic acid .A biochemical test to detect monosaccharide (reducing) sugars in solution, devised by the Swedish physician C. T. Barfoed (1815–99). Barfoed's reagent, a mixture of ethanoic . Principle: In Barfoed’s test, the copper ion in the solution oxidizes the reducing monosaccharide to form a carboxylic acid and copper (I) oxide, resulting in the formation of a red coloured . Principle: Barfoed’s test is a simple and rapid test used for the identification of monosaccharides. In this test, a sample is heated with Barfoed’s reagent (a mixture of copper acetate and acetic acid) in a boiling water bath. Monosaccharides (such as glucose, fructose, and galactose) reduce the copper ions in the reagent to form a red . Principle of barfoed’s test: When barfoed reagent mix with solution of monosaccharide or disaccharide, and heated in boiling water bath, they react and crystal precipitate is formed. Copper . In principle, for all these methods, a (salt-free) solution containing carbohydrate material is treated with a specific reagent, generating a characteristic-colored reaction product that is proportional to the sugar concentration. . 4.3.7 Barfoed’s Test. Barfoed’s test is used to detect the presence of reducing monosaccharides in solution .
How to perform the test: One ml of a sample solution is placed in a test tube. Three ml of Barfoed's reagent (a solution of cupric acetate and acetic acid) is added. The solution is then heated in a boiling water bath for three minutes. A positive test is indicated by: The formation of a reddish precipitate within three minutes.
Principle. Barfoed’s test reaction is based on the reduction of cupric acetate by reducing monosaccharides and reducing disaccharides. The free aldehyde and ketone groups of monosaccharide reduce copper sulfate to cuprous oxide and give red precipitates. Reagent. To 450 mL of boiling water, add 24 g of copper acetate. Tollens’ test also referred to as silver-mirror test is a qualitative laboratory test used to distinguish between aldehyde and ketone. The test is named after a German chemist, Bernard Tollens who discovered the test. The test involves use of Tollens’ reagent which must be prepared immediately prior to its use as an explosive substrate can .
Principle of Seliwanoff’s test. The reagent of this test consists of resorcinol and concentrated HCl. The acid hydrolysis of polysaccharides and oligosaccharides yields simpler sugars. Ketoses are more rapidly dehydrated than aldoses. Ketoses undergo dehydration in the presence of concentrated acid to yield 5-hydroxymethyl furfural.
Barfoed’s test makes use of Barfoed’s solution, which contains copper acetate in the dilute acetic acid with a pH of 4.6. Principle: In Barfoed’s test, the reducing monosaccharide is oxidized by the copper ion in the solution to form a carboxylic acid and copper (I) oxide, which results in the formation of a red-coloured precipitate .principle of barfoed test Barfoed's test The Barfoed’s test is a chemical technique that determines whether or not a sample contains simple sugars. Barfoed’s reagent is a particular liquid that we can mix with the sample and heat to examine if the liquid changes color. If the sample becomes reddish-brown in color, it indicates the presence of monosaccharides, which are simple sugars. Principle of Bial’s Test. This test is based on the principle that under hydrolysis pentosans are hydrolyzed into pentoses. Further, pentoses are dehydrated to yield furfural, which in turn .
Benedict’s Test Principle When a reducing sugar is subjected to heat in the presence of an alkali, it gets converted into an enediol (which is a relatively powerful reducing agent). Therefore, when reducing sugars are present in the analyte, the cupric ions (Cu 2+ ) in Benedict’s reagent are reduced to cuprous ions (Cu + ).

Procedure of Tollens’ test. Take two clean, dry test tubes and add 1 ml of the test sample in one test tube and 1 ml of distilled water in another as blank. Add 2 ml of Tollen’s reagent to both the test .
Procedure of Anthrone Test. Pipette out different volumes (50 µl, 100 µl, and so on) of glucose solution from the supplied stock solution (200µg /ml) into a series of test tubes and make up the volume to 1 mL with distilled water. Take a tube labeled as one as blank containing 1ml of just distilled water and the rest of the tubes labeled 2 .A biochemical test to detect monosaccharide (reducing) sugars in solution, devised by the Swedish physician C. T. Barfoed (1815–99). Barfoed's reagent, a mixture of ethanoic (acetic) acid and copper (II) acetate, is added to the test solution and boiled. If any reducing sugars are present a red precipitate of copper (II) oxide is formed.
Benedict’s test is a chemical test that is used to check for the presence of reducing sugars in an analyte. Hence, simple carbohydrates that contain a free ketone or aldehyde functional group can be identified using this test. The benedict’s test for reducing sugars is based on the benedict’s reagent, which is also known as Benedict’s .
Molisch’s Test Procedure. 2-3 drops of Molisch’s reagent must be added to a small amount of the analyte in a test tube and mixed well. Now, a few drops of concentrated sulphuric acid must be added drop-wise along the walls of the test tube to facilitate the formation of a layer and avoid mixing. The development of a purple ring at the layer . Procedure of Fehling’s Test. Take 1 ml of a given sample in a clean, dry test tube. The concentration of the test samples should be 5% (w/v). Take control of 1 ml of distilled water in another tube. Add about 2-3 drops of Fehling’s reagent to both the tubes and mix them in a vortex. Keep the test tubes in the water bath for 1-2 minutes.
Creamberryfairy,free videos, latest updates and direct chatPlanetSuzy - Download High Quality 4k Ultra Full HD Porn Clips. Search Advanced Search. . Scarlet Chase - Latex Boot Licking and Fucking UltraHD/4K. Latex Fetish / 4K Ultra HD. Download movie. 0. Abuse; 216; 0; 30-09-2023, 03:03; Scarlet Chase - Fuck In Latex And Boots FullHD.
principle of barfoed test|Barfoed's test